Some of the most important reactions in synthetic chemistry and nature involve the transfer of a group from a metal center in a higher oxidation to a reactive substrate in a lower oxidation state. This process is well studied for reactions such as epoxidation and cyclopropanation, but not nearly as thoroughly for aziridination. Aziridination is the nitrogen analog of these reactions and involves transfer of a metal imide (M=N-R) fragment to an alkene substrate (C2 + N1 approach) (A). This reaction necessitates the stabilization of a high valent metal ligand multiple bond, which can be difficult with many typical ligand systems (i.e. porphyrins).
Our research in the Jenkins group involves design of new ligands that circumvent the shortcomings of traditional ligands. To achieve this goal, we employ N-heterocyclic carbenes (NHCs) in the formation of macrocyclic tetra-NHC complexes. While isostructural to porphyrins, the chelating NHCs are able to promote stabilization of higher oxidation states on the metal. We found that several complexes are able to affect catalytic aziridination at with a variety of alkenes and azides, including unfunctionalized substrates (B and C). These results show work towards an atom economical and environmentally friendly method of aziridination, by using abundant iron and only producing N2 as a byproduct. We have also shown similar improved reactivity with chromium systems.
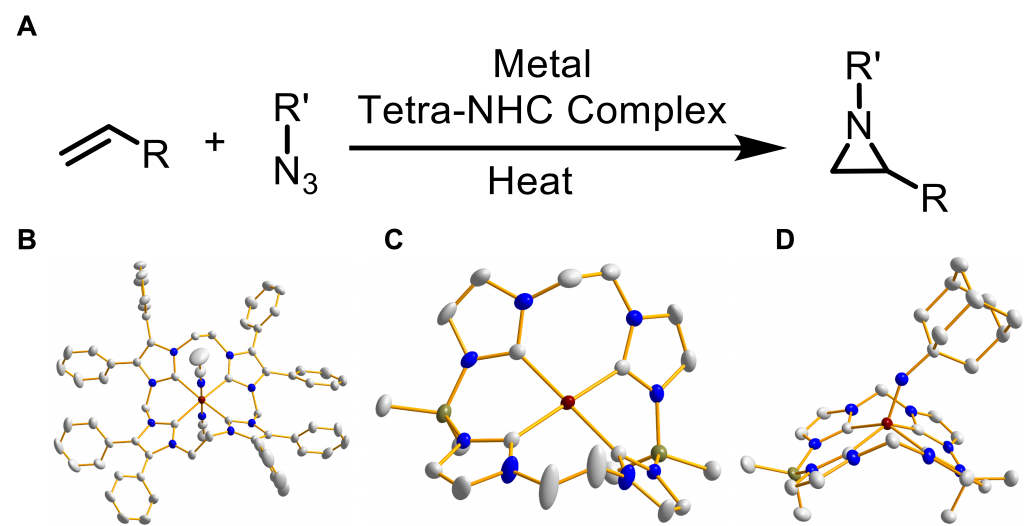
Figure 1: Summarization of Jenkins group carbene project research. A: Typical aziridination reaction. B and C: Catalytically active iron(II) complexes. D: Isolated iron(IV) imide.
Calculations performed by the Roy group show that this reaction goes through an iron imide intermediate. The reaction with alkene is the rate limiting step, which necessitates excess alkene for aziridination. We have been able to observe isolated imides (D) and characterize them, but have not shown that these complexes are catalytically active. Azides can react further with imides to form catalytically inert metallotetrazenes. Current work in the Jenkins’ group involves overcoming limitations based on the proposed mechanism by modulating ligand design and making chiral variants of the macrocycles for chiral tetra-NHCs. The new chiral ligands are a new type of ligand and will be a critical development as there are very few examples of catalytic C2 + N1 aziridination to form chiral aziridines.
Notable Publications:
Synthesis of Aziridines from Alkenes and Aryl Azides with a Reusable Macrocyclic Tetracarbene Iron Catalyst. Cramer, S. Alan; Jenkins, David. M. J. Am. Chem. Soc. 2011, 133, 19342-19345.
Synthesis of Fully Aliphatic Aziridines with a Macrocyclic Tetracarbene Iron Catalyst, Chandrachud, Preeti P.; Bass, Heather M; Jenkins, David M. Organometallics, 2016, 35, 1652–1657.
Catalytic aziridination with alcoholic substrates via a chromium tetracarbene catalyst, Keller, C. Luke; Kern, Jesse L.; Terry, Bradley D.; Roy, Sharani; Jenkins, David M., Chem Commun, 2018, 54, 1429-1432.
Unprecedented Five‐Coordinate Iron(IV) Imides Generate Divergent Spin States Based on the Imide R‐Groups, Anneser, Markus R.; Elpitya, Gaya R.; Townsend, Jacob; Johnson, Elizabeth J.; Powers, Xian B.; DeJesus, Joseph D.; Vogiatzis, Konstantinos D.; Jenkins, David M. Angew. Chem. Int. Ed. 2019, 58, 8115-8118.
Elucidation of the Reaction Mechanism of C2+ N1 Aziridination from Tetracarbene Iron Catalysts, Isbill, Sara B.; Chandrachud, Preeti P.; Kern, Jesse L.; Jenkins, David M.; Roy, Sharani. ACS Catal. 2019, 9, 6223-6233.